
- #Energy state and time in heisenberg principle how to
- #Energy state and time in heisenberg principle free
However, if we took $n=0$, we would have a nonsense imaginary answer (of course, the wave function is identically zero for $n=0$). Provided that we have an infinite square well with potentialįor higher $n$ the inequality is even stronger. Thus, $\psi=0$ everywhere and is not a physical state in our Hilbert space. However, there is no nonzero linear function that can satisfy the necessary boundary conditions. In other words, they would satisfy the eigenvalue equation $$\hat=0$$Īnd thus is linear. If what you said were true, then the states of definite energy would also be the states of definite momentum. There's a simple and (in my opinion) instructive way to see this. For example, the values of the energy of a bound system are always discrete, and angular momentum components have values that take the form m, where m is either an integer or a half-integer, positive or negative.
#Energy state and time in heisenberg principle free
This is true for a completely free particle, but this is no longer true for a particle that is undergoing some interaction (where is the interaction, you ask? Well the fact that it is placed in a box, of course!) The observables discussed so far have had discrete sets of experimental values.

For a start, you seem to say that any definite value of energy would entail a definite value of momentum. There are a couple of assumptions your question makes that aren't strictly true. But this is by no means a rigorous answer.
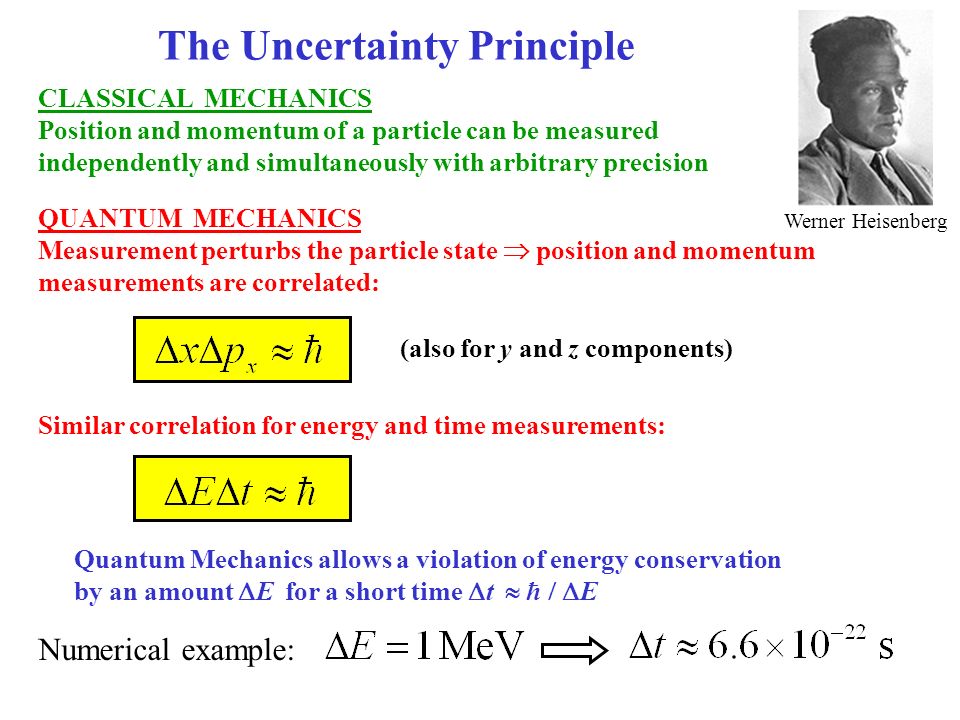
The reason is that the frequency of a state is inversely proportional to time and the frequency connects with the energy of the state, so to measure the energy with good precision, the state.

This started off as comment, but right now I only have the reputation to answer. Nevertheless, the general meaning of the energy-time principle is that a quantum state that exists for only a short time cannot have a definite energy. Which is also a known energy, and so why does this (as well as the other integer values of $n$) does not violate the uncertainty principle? Heisenberg’s uncertainty principle states that there is a fundamental limit to the precision with which certain pairs of physical properties of a particle (complementary. Surely when $n = 1$ we have the energy as Therefore $n$ can only be greater than or equal to one. However, this cannot the case be in an infinite well, as we know the particle should be somewhere in the box by definition. However, I have read somewhere that the reason that the quantum particle cannot have $n = 0$-in other words, $E = 0$-is because by having zero energy we also have a definite momentum with no uncertainty, and by the Heisenberg uncertainty principle this should lead to an infinite uncertainty in the position of the particle.
#Energy state and time in heisenberg principle how to
I understand how to apply Schrödinger's equation and appreciate that energy Eigenvalues can be deduced to be However, I am a bit confused as to how exactly it applies to the quantum mechanical situation of an infinite square well. \langle E\rangle = \frac = \Delta x$.I appreciate the statement of Heisenberg's Uncertainty Principle. If the energy state E only lasts for a brief period of time t, its energy is uncertain.

The expectation value of the energy is therefore The Heisenberg uncertainty principle states that. So far I know that the total energy of the particle will be I'm quite confused about how to handle the absolute value in the potential. I'm currently trying to solve a problem that involves estimating the minimum energy of a particle in the potential:


 0 kommentar(er)
0 kommentar(er)
